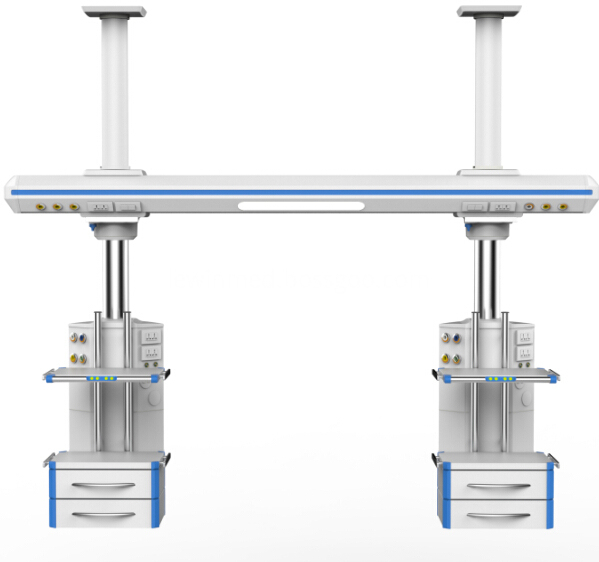
The cross arm and control cabinet of the tower crane adopt the imported high-strength aluminum alloy ofpatent technology for the one off extrusion molding, ICU Bridge and the surface undergoes the primary oxidationtreatment.
ICU Bridge Icu Bridge,Icu Bridge Pendant,Portable Supply Unit,Tower Crane Shandong Lewin Medical Equipment Co., Ltd. , https://www.operatinglight.nl
Due to the sensitivity of the time, the head of R&D of an antibacterial drug manufacturer repeatedly told reporters after anonymity that the "Measures" were unusually strict and it was estimated that the development of new drugs for domestic antibacterial drugs would cause greater difficulties.
"I believe that after the "Measures" have been issued, relevant companies will become more cautious about the new project, and may even give up part of the unsuitable projects." The person in charge said frankly that despite the pains, the "Measures" will improve domestic antibacterial activities. The quality of drug research and development.
Need to mobilize R&D initiative
As the industry has agreed, the introduction of the "Measures" is expected to delay the current antibiotic resistance process through rational use. However, the key to truly responding to drug-resistant bacteria is still continuous research and development.
Executives of an antibiotic production company in the south said that even newly researched antibacterial drugs are often listed on the market in less than a few years and clinically there will be drug resistant bacteria, leading to a lower return on investment for enterprises, but this does not directly constitute a slower R&D. reason. As for the core reason for the slowdown in R&D, the executive has not directly pointed out, but simply stated that the pharmaceutical industry is an industry dominated by intellectual property R&D. From a global perspective, new drug development is relatively active, and not every category has seen a slowdown in antimicrobial drug development.
"Actually, the emergence of drug-resistant bacteria means that there is a demand for research and development of new drugs, and drug resistance can be said to provide reasons for the development of new drugs for antimicrobial drugs," said the executive.
Some researchers believe that the reason why the research and development fever of antibacterial drugs is relatively low in recent years is due to changes in the spectrum of human diseases and changes in the focus of the pharmaceutical market. They have evolved from anti-infective, digestive and hypertensive drugs in the 1970s and 1980s. For today's anti-tumor drugs, chronic disease medication. According to the statistics of the SFDA Southern Medicine Economic Research Institute, the global anti-infection market growth trend has slowed down, with an average annual growth of 16.55% from 2005 to 2008, and from 2008 to 2012, the growth rate may drop to 5.21%.
“Enterprises need to have incentives to research and develop new drugs. However, with the increasingly stringent drug regulation, especially with more and more postmarketing drug evaluations, the pace of research and development of new drugs has slowed down.†Technical Director of Shanghai Adverse Reaction Monitoring Center Wen Min told reporters that the lack of economic benefits is one of the reasons for the delay in the development of antimicrobial drugs.
"The market for antineoplastic drugs and cardiovascular drugs is large and rapid, and pharmaceutical companies are naturally more willing to invest funds in these two projects," Du Wenmin said.
Du Wenmin believes that due to the rapid emergence of drug-resistant bacteria, it is difficult for antibacterial drug companies to recover the cost of R&D. Therefore, it is hoped that the government will be able to tilt its pricing mechanism and tendering system to encourage new types of systems and mechanisms. Antibacterial drug research and development, mobilize the enthusiasm of the company.
Regarding Du Wenmin’s proposal, the above-mentioned executives also expressed their approval: “If the new drug fails to enter the directory of the “Administrative Measures,†the hospital sales cannot be carried out, and it will be impossible to enter the medical insurance catalog. The rate of return on investment will be greatly reduced. It is expected that there will be adjustment channels in the future, and restrictions on some new listed antibiotics will be appropriately released."
Call for national funding support
Right now, the abuse of antibiotics has led to the emergence of new bacterial drug resistance problems. New unknown viruses are also looking for opportunities to invade humans. The combination of the two dangers makes the development of new antibacterial drugs more urgent, and guiding the rational development of antimicrobial drugs has become a common problem in the industry.
Apart from the benefit factors, in view of some researchers, another reason for the delay in the development of antibacterial drugs is the lack of substantial breakthroughs in basic research such as therapeutic pharmacology and antibacterial mechanisms.
To this end, Du Wenmin suggested that for some basic research, such as bacterial resistance mechanisms, bacterial sensitive targets, etc., need to increase national public funding for its investment. As far as international practice is concerned, purely basic research on antibacterial drugs is generally based on the state. "The new revolutionary research should be based on the state, and the state should provide special funds for investment." Du Wenmin believes that only by achieving a fundamental theoretical breakthrough, antibacterial drug research and development will make great progress.
For individual drug R&D, Du Wenmin thinks it can rely on companies because “the impulse of R&D products is very strongâ€. However, if it is necessary to start from the selection of lead compounds for new drug research and development, large investment, long time, and high risk, it is difficult for domestic pharmaceutical companies to rely on their own strength to achieve. Therefore, Du Wenmin proposed that a special fund should be set up at the national level to start R&D from the chemical structure screening. Through strict review of the company's declaration materials and qualifications, it should give sufficient funding for research and development proposals, and at the same time strictly monitor the use of funds.
"If we can support the country and really improve our ability to innovate, we will have a great chance of becoming a "bomb bomb" once a breakthrough new drug is developed. Enterprises will have the ability to develop blood and enter a virtuous cycle of continued investment in R&D." Wen Min is depicted.
For enterprises, the national support horn has already sounded. In the "12th Five-Year Plan for Major New Drugs Creation" major project recently announced by the Ministry of Health and the Ministry of Health, the Ministry of Health issued a "Key Research Project for New Drug Research and Development in the 12th Five-Year Plan." Supporting enterprises as the mainstay, a multidisciplinary combination of production, education and research. The goal of this project is to break through and master a batch of new core key technologies to support and lead the research, development, and industrialization of innovative drugs in China.
Me-too develops odds of geometry
Even though the current status of secondary development of domestic antibacterial drugs is not satisfactory, Du Wenmin also agrees that research on new indications of antibacterial drugs and other minor changes (such as compound compatibility and dosage form improvement) are currently meeting domestic medical needs. One of the ways.
Less investment in secondary development, which is why most domestic companies are willing to carry out. However, a person in charge of the pharmaceutical company told reporters that he believes there is little chance of secondary development of antibiotics. "The development of new indications for existing antibiotics is not the direction." The responsible person pointed out that the main reason is that secondary development will not substantially change the chemical structure of the original drug, and the applicable bacterial population will not change.
However, from the perspective of the winds that were revealed before, the implementation of the "Measures" may mean that the approach to compound development has narrowed substantially. In terms of development, "me-too" drugs are less risky, cost and cycle time will be better, and investment will be less. Therefore, domestic companies will still have more layouts due to their R&D capabilities and risk bearing capacity." Me-tooâ€.
"The cost and risk of the development of 'me-too' drugs will be smaller, but it is hard to say how much progress the drug has made in terms of its actual efficacy, safety, and economy," said the person in charge of the company.
Pneumatic brake plus mechanical friction damping brake
The imported electrical machine is adopted, and the electric perpendicular moving up and down.The gas pipeline, power supply and computer communication line are separately arranged withoutinterference.The imported (GENTEC) brand German standard gas terminal (over 20,000 of inserting and pulling out) is adopted.The ICU bridge with high bearing capacity with suspension the cavity mirror car system.The horizontal rotation function can accurately position without excursion.
Antibacterial drug New Deal or new antibacterial drug
Business News Agency August 3 hearing as of July 31, the industry has long been popular in the "clinical application of antibiotics management approach" (hereinafter referred to as "measures") has not yet formally notified the implementation, however, the "measures" impact on the antimicrobial industry chain The flaw has spread to research and development.