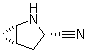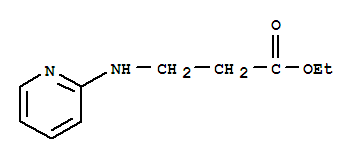(I) Product Features
Ginkgo biloba kernels and hawthorn as raw materials, the production of nutritious products, with Runfei Dingchuan, Shibuya fine belt, rule children with enuresis, consumer product, stomach and spleen, blood pressure, prevention of cardiovascular disease and other health effects.
(II) Main raw materials
Silver almonds, hawthorn, white sugar, citric acid, sodium alginate, sucrose esters.
(III) Major equipment
Ovens, soaking tanks, beaters, colloid mills, homogenizers, high temperature flash sterilizers, filling and sealing machines, retort pots.
(IV) Process flow
(1) Silver almonds → Shelled → Leather clothing → Baking → Soaking → Beating → Colloid mill → Homogeneous
(2) Hawthorn → Selection → Washing → Crushing → Leaching → Filtration
(3) Batching the product of (1) and (2) → homogenization → instantaneous sterilization → filling and sealing → sterilization, cooling → finished product
(five) operation instructions
(1) Silver almond processing
1 Preservation of silver almond raw material: Silver almond, also known as ginkgo, will be used to manually remove the ginkgo after removing the bad fruit and manually remove the leather garment. If the production volume is large, the ginkgo can be dried first, and then peeled with a roller sheller.
2 Baking: Place the peeled nuts on a tray and bake them in an oven. The thickness of the nuts on the tray does not exceed 3 cm. The temperature starts at 85-90°C for 1.5 hours, and then it is placed at 115-120°C. The time is 2 hours until the white nuts are yellow in color or dark in color, and the odor is baked scented type.
3 Soaking: According to the weight ratio of 1:3, the roasted nuts and the treated softened water are soaked. The water temperature is 65-70°C and the time is 2.5 hours.
4 Beating and colloidization: Beating with a single-channel horizontal beater, beating and then diluting the pulp juice with demineralized water for 1 time, then grinding with a colloidal colloid mill for 15 minutes, and adjusting the particle size to 5 microns or less.
5 Homogeneous: The material temperature is about 35°C and homogenized with a pressure of 25 MPa.
(2) Hawthorn processing: after removing the dried rot fruits, pests and diseases, etc., the hawthorn is washed twice with fresh water, broken into small pieces with a hammer mill, and then placed in a stainless steel tank and added at about 85°C. The demineralized water was just immersed in the raw materials, and the temperature was maintained at about 85°C. After 2 hours, the juice was filtered out. Then, the remaining filter residue was added with softening water at about 85°C that was just immersed in the filter residue, and the filtrate was filtered after 2.5 hours. The two filtrates were combined and filtered with a flannel for later use.
(3) Treatment of other raw materials: Dissolve 75% syrup, add 0.5% citric acid to facilitate the removal of impurities in the sugar and filter it with a sugar filter. Dissolve 5% sodium alginate and 2% sucrose ester solution.
(4) Formulation: The following proportions were formulated: 500 g of white juice, 1000 g of hawthorn juice, 250 g of 75% syrup, 30 g of 5% sodium alginate, and 50 g of 2% sucrose ester.
(5) Homogenization: The homogenized material is homogenized twice at a pressure of 16-18 MPa.
(6) Instantaneous temperature rise: The material is heated to above 85°C with a high-temperature instantaneous sterilizer, and the material is hot-filled and hot-sealed.
(7) Sterilization: The sealed cans were sterilized in hot water at 95°C or more for 15 minutes.
(VI) Product Quality Index
1. Sensory Index
Color: Light pink or orange-yellow; Odor: Baked fresh and sweet and sour mixed flavor; Sweet and sour taste, full after taste, with a unique flavor of baked ginkgo; Uniformity: A stable and uniform form; slightly viscous Sense; Impurities: No visible foreign matter, impurities.
2. Physical and chemical indicators
Vacuum degree ≥ 0.03 MPa; soluble solids ≥ 12% (refractive method); total acid (calculated as citric acid) ≥ 0.3%; protein ≥ 0.25%; pectin ≥ 0.08%; Vc ≥ 1.5 mg/100 g.
APIs & Intermediates
Intermediates of Cladribine, Carvedilol, Lurasidone, olmesartan,
Risedronate Sodium, Atazanavir, Saxagliptin,
Dabigatran,Dapoxetine,Cefixime,Ceftaroline fosamil and etc.
In the short span of time, we have emerged as most promising
pharmaceutical intermediates manufacturers, chemical intermediates and
bulk drug intermediates suppliers. Our consistent supply, quality
products and dedication towards clients have opened up many
international avenues for our growth.
In addition, the company also can follow the customer's product needs custom synthesis services
MAIN API PRODUCTS USP/BP
|
PRODUCT NAME
|
CAS NUMBER
|
SPEVIFICATION
|
|
Azithromycin
|
117772-70-0
|
BEP
|
|
Cefpirome Sulphate sterile
|
84957-29-9
|
USP JP16
|
|
Ceftriaxone Sodium (Sterile)
|
104376-79-6
|
USP31
|
|
Cefotaxime
|
64485-93-4
|
USP30
|
|
Ciprofloxacin HCL
|
85721-33-1
|
USP/BP
|
|
Gentamicin sulphate
|
1405-41-0
|
BP
|
|
Levofloxacin
|
100986-85-4
|
USP27
|
|
Lincomycin Hydrochloride
|
859-18-7
|
EP6.0
|
|
Moxifloxacin Hydrochloride
|
186826-86-8
|
USP31
|
|
Tigecycline
|
220620-09-7
|
USP
|
|
Linezolid
|
165800-03-3
|
EP
|
|
Dexamethasone
|
50-02-2
|
USP/BP/EP
|
|
Methylprednisolone
|
83-43-2
|
USP/BP/EP
|
|
Dexketoprofen trometamol
|
156604-79-4
|
BP2008
|
|
Ibuprofen
|
15687-27-1
|
BP
|
|
Metamizol
|
68-89-3
|
DAB
|
|
Sulindac
|
38194-50-2
|
USP/BP/EP
|
|
Naproxcinod
|
163133-43-5
|
USP28
|
|
Tripelennamine Hydrochloride
|
154-69-8
|
USP28
|
|
Itraconazole
|
84625-61-6
|
USP/BP
|
|
Cytarabine
|
147-94-4
|
USP31
|
|
Leucovorin Calcium
|
1492-18-8
|
USP32
|
|
Valsartan
|
137862-53-4
|
USP30
|
|
Telmisartan
|
144701-48-4
|
USP31
|
|
Rosuvastatin Calcium
|
147098-20-2
|
USP/BP
|
|
Pitavastatin Calcium
|
147526-32-7
|
USP/BP
|
|
Fluvastatin
|
93957-54-1
|
USP31
|
|
Vinpocetine
|
42971-09-5
|
EP6.0
|
|
Atazanavir
|
198904-31-3
|
BP
|
|
Rosiglitazone
|
122320-73-4
|
USP30
|
|
Esomeprazole Magnesium
|
161973-10-0
|
USP/BP
|
|
Topiramate
|
97240-79-4
|
USP31
|
|
Fexofenadine HCl
|
153439-40-8
|
Inhouse
|
|
Bosentan
|
147536-97-8
|
Inhouse
|
|
D-Cysteine
|
921-01-7
|
Inhouse
|
|
D-Phenylalanine
|
673-06-3
|
Inhouse
|
|
Linagliptin
|
668270-12-0
|
Inhouse
|
|
Rivaroxaban
|
366789-02-8
|
USP
|
|
Saxagliptin
|
361442-04-8
|
USP
|
|
Vildagliptin
|
274901-16-5
|
USP
|
Major Pharmaceutical Intermediates
|
Items Descripation
|
Structure
|
Application
|
MICA ESTER
CAS No: 246035-38-1
Purity: ≥98%
|
|
For Cefixime
|
EHATA
CAS No: 64485-82-1
Purity: ≥98%
|
|
For Ceftazidine
|
2-Chloroadenine
CAS No: 1839-18-5
|
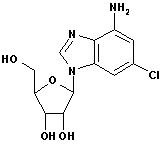
|
For Cladribine, Fludarabine et al
|
Bicyclo(2,2,1)Heptane-2,3-di-exo-carboximide
CAS No: 14805o-29-9
|
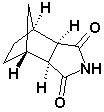
|
For Lurasidne
|
(R,R)-1,2-Bis(methanesulfonyloxy methyl)Cyclohexane
CAS No: 186204-35-3
|
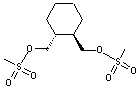
|
For Lurasidone
|
3-(Piperazin-1-yl)benzol[d] isothiazole
CAS No: 87691-87-0
|
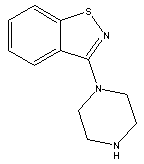
|
For Lurasidone
|
Trityl olmesartan
CAS No: 144690-92-6
Purity: ≥98%
|
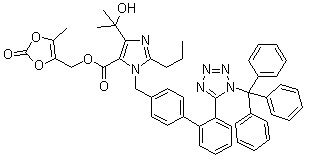
|
For olmesartan
|
3-Acetyl Pyridine
CAS No: 350-03-8
|
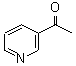
|
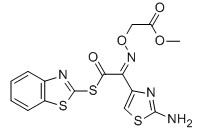
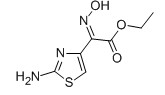
For Risedronate Sodium
|
3-(AceticAcid)pyridine HCL
CAS No: 6419-36-9
|
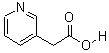
|
For Risedronate Sodium
|
Risedronic Acid
CAS No: 105462-24-6
|
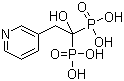
|
For Risedronate Sodium
|
3-Hydroxy-1-adamantyl-D-Glycine
CAS No: 709031-29-8
|
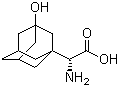
|
For Saxagliptin
|
(1s,3s,5s)-3-(aminocarbonyl)-2-azabicyclo(3,1,0) hexane-2-carboxylic acid tert-butyl ester
CAS No: 361440-67-7
|
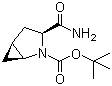
|
For Saxagliptin
|
(S)-N-Boc-3- hydroxy-adamantylglycine
CAS No: 361442-00-4
|
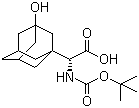
|
For Saxagliptin
|
2-Azabicyclo[3.1.0] hexane-3-carbonitrile, (1s,3s,5s)-
CAS No: 866083-42-3
|
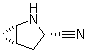
|
For Saxagliptin
|
Ethyl 3-(pyridin-2-ylamino) propanoate
CAS No: 103041-38-9
|
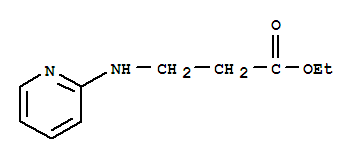
|
For Dabigatran
|
N-(4-Cyanophenyl) glycine
CAS No: 42288-26-6
|
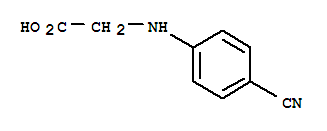
|
For Dabigatran
|
4-methylamino-3-nitrobenzoic Acid
CAS No: 41263-74-5
|
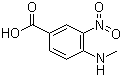
|
For Dabigatran
|
S-3-Amino-3-phenylpropanoic acid ethyl ester HCL
CAS No: 167834-24-4
|
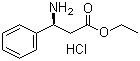
|
For Dapoxetine
|
(S)-3-Amino-3-Phemylpropan -1-ol
CAS No: 82769-76-4
|
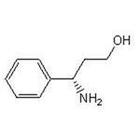
|
For Dapoxetine
|
(S)-3-Dimethylamino-3-Phemylpropanol
CAS No: 82769-75-3
|
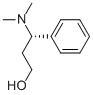
|
For Dapoxetine
|
4-{4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-1-butynil}-α,α-dimethyl benzene acetic acid
CAS No: 832088-68-3
|
|
For Fexofenadine HCl
|
Methyl 2-(4-(4-chlorobutanoyl)phenyl)-2-methylpropanoate
CAS No:154477-54-0
|
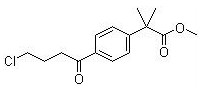
|
For Fexofenadine HCl
|
5-Bromo-2-chlorophenyl)(4-ethoxyphenyl)methanone
CAS No 461432-22-4
|
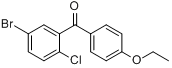
|
For Dapagliflozin
|
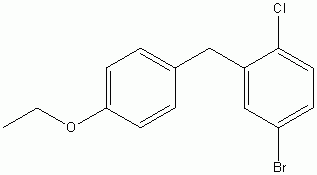
4-(5-Bromo-2-chlorobenzyl)phenyl ethyl ether
CAS No :461432-23-5
|
|
For Dapagliflozin
|
Mica Ester,Pharma Intermediates,Ciprofloxacin Hcl Uses,Active Pharmaceutical Ingredients
NINGBO VOICE BIOCHEMIC CO. LTD , https://www.pharma-voice.com