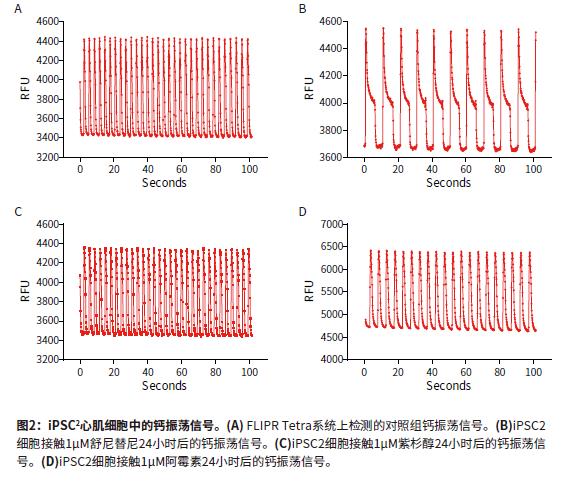
Introduction The continued increase in drug availability and safety screening costs has led to a need for innovative technologies to enable early detection and analysis of characteristics during drug discovery. The FDA is developing guidelines for compound testing to ensure the safety of the drug so that the drug does not need to be withdrawn due to adverse effects, such as blocking the heart's hERG channel and causing symptoms such as torsades degenerative ventricular arrhythmias. This direction is known as the comprehensive in vitro arrhythmia test or the CiPA project. Cardiomyocytes that induce pluripotent stem cell (iPSC) differentiation are widely used in in vitro model systems because of their combined effects on assessing cardiac function and safety. The results of calcium signal oscillations can reflect changes in intracellular calcium ion concentration, allowing the detection of signals using calcium sensitive dyes such as the EarlyToxTM fluorescent dye kit. ScreenWorks® Pro software analyzes the frequency of myocardial cell pulsation peaks, peak amplitude peak width of 10%, and other parameters to describe compounds affecting cardiomyocytes in a high-throughput format. Stem Cell CDI (Cellular Dynamics International) has developed an enhanced version of iPSCs-differentiated cardiomyocytes that recover faster after recovery than previous versions of iPSCs. Such improvements have resulted in a shortened growth time of 5-7 days in the FLIPR Tetra high-throughput real-time fluorescence detection and analysis system for detecting the effects of compounds on beating cardiomyocyte calcium transient signals. Figure 1 is an example workflow. Here we describe the detection of some cancer compounds, some of which are known to affect the hERG channel, and other compounds known to block the hERG channel. Advantage • Reduce cell culture time, improve workflow and quality of experiments • Identify compounds that may cause safety problems earlier in drug discovery • ScreenWorks Peak Pro software simplifies data analysis material • iCell® Cardiomyocytes2 kit, including cells, plating medium and maintenance medium (Provided byCellular Dynamics, International) Prepare iCell cardiomyocyte perforated plate The 384-well black-walled perforated plate was coated with 0.1% gelatin under the guidance of the iCell Cardiomyocyte Use Guide. After resuscitation, iCell cardiomyocytes were plated at a density of 8000 cells per well in 25 μL of medium per well. The cells were cultured for 24 hours at 37 ° C, 5% CO 2 , and 100% humidity. The cells form a monolayer and are replaced with a maintenance medium. The cells were replaced with maintenance medium every other day. On day 5, the cells began to be synchronized and could be used for experiments. Prepare compound plates for screening detection For ease of screening assays, the compound was dissolved in 100% DMSO to a 10 mM mother liquor. Maintain medium dilution mother liquor to a detection concentration of 10-100 μM. The diluted compound solution was added to each well 24 hours before the test using the FLIPRTetra system. The final concentration of DMSO in the medium was 0.15% (v / v). Concentrations ranged from 30 or 100 μM log dilutions of each concentration of compound to two (n = 2) or three (n = 3) assays. Observing calcium oscillations using the FLIPR Tetra system Four hours before the experiment, logarithmic dilutions of various compounds starting at a final concentration of 30 μM were added to iPSC cardiomyocytes. A 25-μL volume of EarlyTox cardiomyocyte cytotoxicity detection dye dissolved in maintenance medium twice before the test was added to each well and incubated at 37 ° C, 5% CO 2 . When the cells are removed from the incubator, changes in calcium ion concentration change as the cells oscillate, causing changes in the fluorescent signal recorded by the FLIPR Tetra system. The experimental scheme and parameter settings in the ScreenWorks working software are shown in Table 1. Data analysis used the ScreenWorks PeakPro software module and plotted in GraphPad Prism 6. Effect of compounds on peak parameters of myocardial cell pulsation Detection and recording of calcium oscillation signals were performed 24 hours after compound addition using the FLIPR Tetra system. iPSC2s was tested in the same manner as previous versions of iPSCs, and the peak frequency indicated by the calcium sensitive dye was detected by forming a gap junction and synchronizing oscillations. The new cells were tested 4-5 days earlier than previous versions of the cells, and the screening workflow was significantly improved due to their lower risk of contamination and a shorter overall time period and similar results. Antitumor compounds used in experimental studies include: sunitinib, imatinib and staurosporine (compounds that are not used clinically for toxicity reasons) are kinase inhibitors; busulfan, an alkyl sulfonate Acid; doxorubicin, an anthracycline; etoposide, a topoisomerase II inhibitor; and paclitaxel interferes with the production of tubulin. After 24 hours of compound addition, some of the calcium oscillation signal changes were significantly different from the control group (Figure 2). Sunitinib has a lower oscillation frequency and a peak width at the 10% peak height is significantly greater than the control group. Other anticancer compounds with similar calcium oscillation results include Imatinib and Staurosporine. These three compounds are involved in blocking the hERG channel. The hERG gene encodes a potassium channel that contributes to the recovery of heart beats, which may cause long QT syndrome or torsades degenerative ventricular arrhythmias. Paclitaxel, busulfan and etoposide are not known to have hERG activity, and there is no similar calcium oscillation slowing effect, nor the effect of increasing peak width at 10% peak height. The dose-response curve showed a significant decrease in peak frequency after exposure to doxorubicin and sunitinib for 24 hours. Paclitaxel is also a compound that does not affect the peak frequency (Figure 3). In addition to anticancer compounds, the known hERG blocker droperidol, an antipsychotic and dopamine receptor blocker; amitriptyline, tricyclic antidepressants and serotonin receptor inhibitors; Cisapride, an antacid, is known to cause long QT syndrome. All three compounds were also tested in the experiment. When iPSC2s was added within 24 hours, the peak frequencies of these three compounds were decreasing and the peak width at the 10% peak height was increased (Fig. 4). A summary of the IC50 values ​​of all compounds tested in the calcium oscillation assay is shown in Table 2. in conclusion iPSC2 cardiomyocytes can be tested within 4-5 days of cell thawing, rather than 10-14 days, improving the workflow for screening assays on the FLIPR Tetra system. ScreenWorks Peak Pro software module can easily confirm the effect of compounds on cardiomyocytes by recording and analyzing calcium oscillation signals, and is an effective tool in the drug screening process. Such an assay can be performed early in the development of the drug to help identify compounds that may have an adverse or beneficial effect on the heart prior to entering the lead compound development process. references: 1. Sirenko, Oksana, et al. “Phenotypic Assays for Characterizing Compound Effects on Induced Pluripotent Stem Cell-Derived Cardiac Spheroids.†ASSAY and Drug Development Technologies, vol. 15, no. 6, 2017, pp. 280‒296., doi:10.1089/ Adt.2017.792. Fire Extinguisher,Automatic Dcp Fire Extinguisher,Co2 Type Fire Extinguisher,Water Mist Extinguisher NINGBO TOMAN IMP. & EXP. CO., LTD , https://www.tdotmfiresolution.com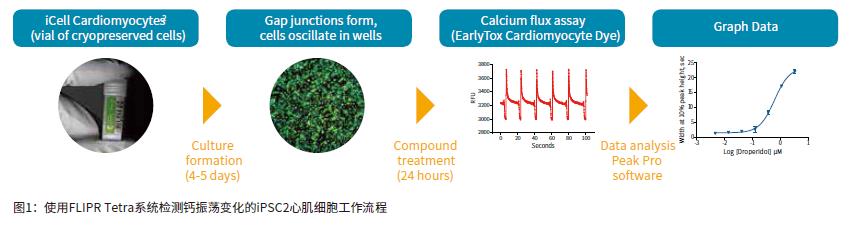
• 0.1% (w/v) gelatin • 384-well black-walled perforated plate (Corning PN 3712)
• EarlyTox Cardiomyocyte Toxicity Assay Kit (Molecular Devices PN R8210)
• Test compound and DMSO (Sigma Aldrich)
• FLIPR Tetra High-throughput Real-Time Fluorescence Detection and Analysis System (Molecular Devices) 



2. Sirenko, Oksana, et al. “Invitrocardiotoxicityassessmentofenvironmentalchemicalsusinganorganotypic human induced pluripotentstem cell-Derived model.†Toxicology and Applied Pharmacology, vol. 322, 2017, pp.60‒74., doi:10.1016/j.taap.2017.02.020.
3. Sirenko, Oksana, et al. "Assessment of beating parameters in human induced pluripotent stem cells enables quantitative invitro screening for cardiotoxicity." Toxicology and Applied Pharmacology, vol. 273, no. 3, 2013, pp. 500‒507., doi: 10.1016/j.taap.2013.09.017.
4. “iCell Cardiomyocytes2 User Guide.†Cellular Dynamics International, Aug 2016.